
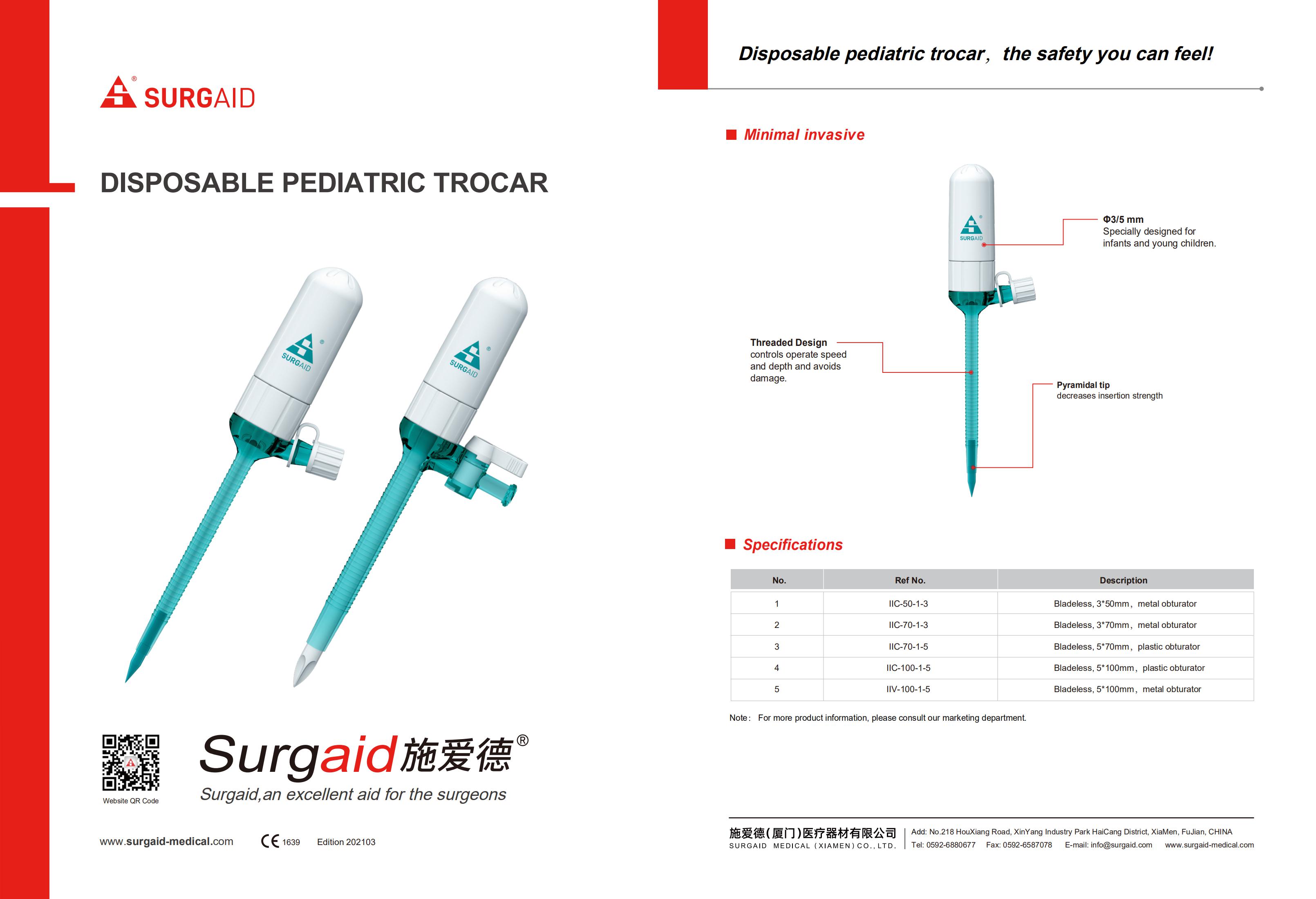
Product Description:
disposable laparoscopic appendectomy trocar (Laparoscopic Optical Trocar) isused in minimally invasive abdominal and gynecologic procedures to establish a path of entry for endoscopic instruments.
Product Application:
Laparoscopic Trocar Set is extensively applied in various laparoscopic surgery including general minimally-Invasive surgery, gynecologycal minimally-Invasive surgery, thoracic or urological endoscopic surgery, and more.
Product Feature:

Dedicated to rehabilitation, Double Medical focus on providing high-quality, affordable products, quick logistics and constant doctors' support.